Which Technique Would You Use to Separate Proteins by Charge
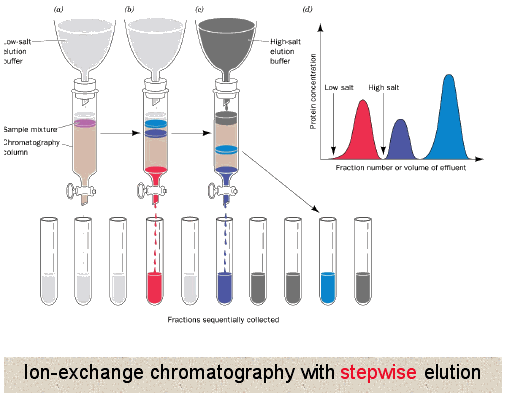
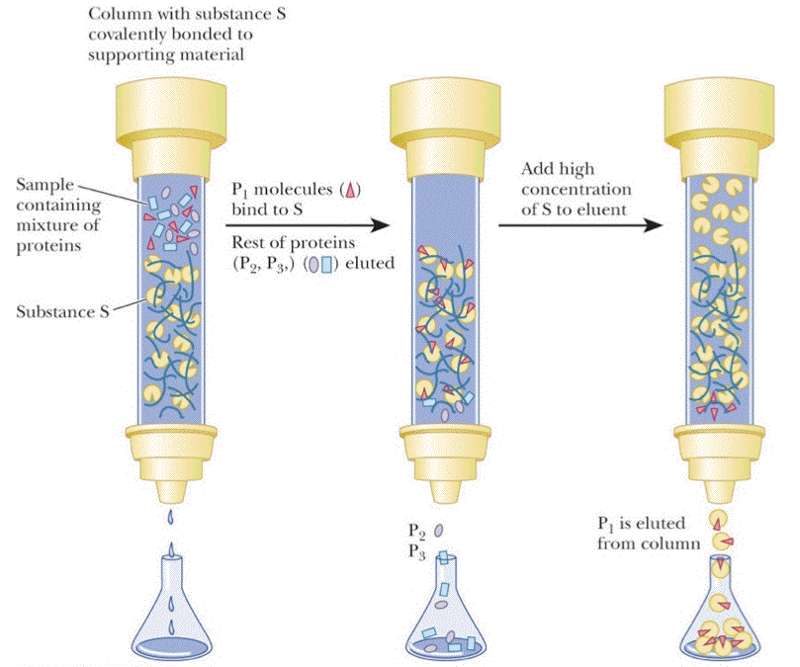
Described both the method used and how it works. Matrix has an ion load opposite to that of the protein to be separated and the affinity of the protein to the column is achieved with ionic ties.
E hydrophobic interaction chromotography A technique that can be used to separate proteins based primarily on their pI isoelectric point is called.
. An electric current is used to move the molecules through a gel or other matrix. What technique would you use to separate. Typically gels made from polyacrylamide are used to separate proteins on the basis their different sizes.
The gel electrophoretic technique used to separate proteins is polyacrylamide gel electrophoresis PAGE. Consequentially proteins of a certain range in size will require a variable volume of eluant solvent before being. The degree of protein purity required depends on the intended end use of the protein.
To determine the size of the. The principle is that smaller molecules have to traverse a larger volume in a porous matrix. Positively charged ion- exchange matrices are called anion-exchange matrices and adsorb.
The gel contains a substance called SDS which has a negative charge. Also useful to isolate and extract DNA fragments of a specific size. IEX is a common protein purification technique in which we separate proteins based on charge.
Pores in the gel or matrix work like a sieve allowing smaller molecules to move faster than larger molecules. Electrophoresis by itself is difficult to separate based on molecular weight. Between specific proteins can affect their functionality in the system.
B Protein B from the rest C. Several techniques for protein purification are used to reach a required purity level. Protein electrophoresis is a standard laboratory technique by which charged protein molecules are transported through a solvent by an electrical field.
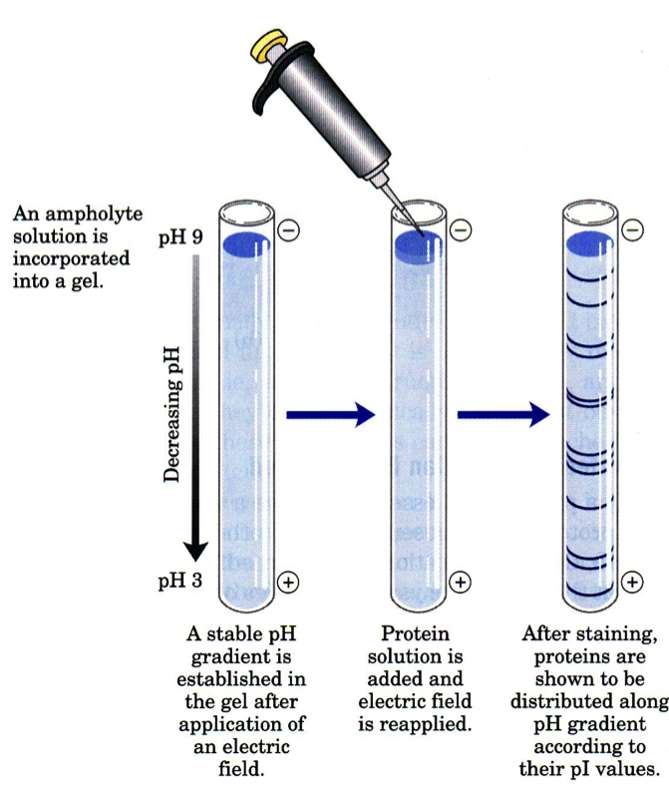
The net charges of the proteins travel towards different ends. Conversely however proteins with very similar pI values but very different sizes can run at the same position. The other principal method of separating proteins by charge is to allow them to migrate along a pH gradient in an electric field coming to rest at the pH at which their net charge is zerothe proteins isoelectric point Pi.
Use gel electrophoresis to separate PCR amplified DNA fragments. The technique is capable of extremely high resolution with proteins differing by a single charge being fractionated into separate bands. Ion exchange chromatography is a technique used to separate molecules or proteins according to their charge.
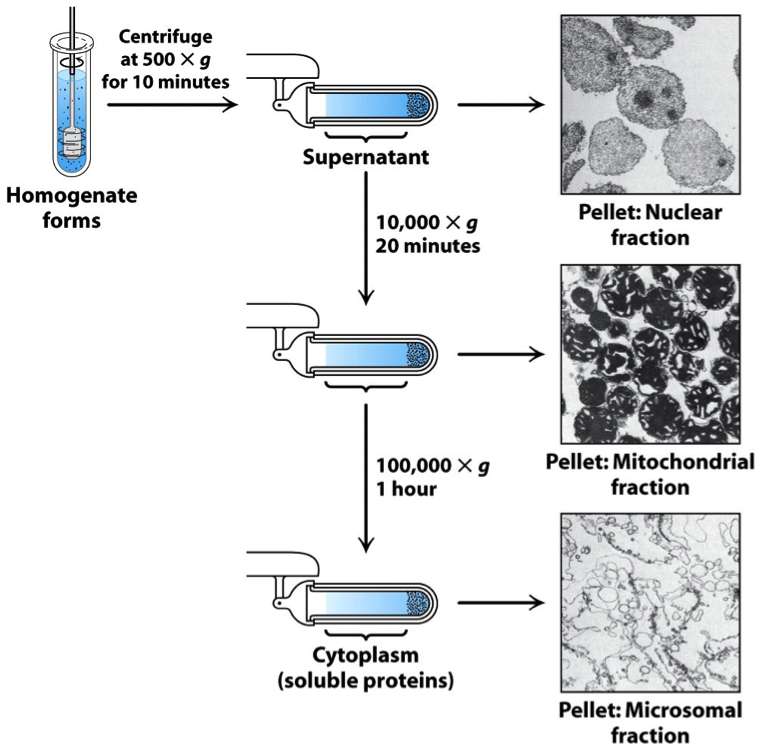
Proteins are separated from the column either by changing pH concentration of ion salts or ionic strength of the buffer solution. This technique is known as size exclusion chromatography. Suggest why proteins are heated before being placed in the electrophoresis gel.
SDS is a detergent that gives all the proteins the same overall negative charge so that when an electric current is applied to the gel separation is only due to the size. Protein A from the rest. Protein Purification Techniques A.
SDS binds to proteins. Electrophoresis uses an electric field applied across a gel matrix to separate large molecules such as DNA RNA and proteins by charge and size. It can also serve to purify proteins for use in further applications.
High-performance liquid chromatography HPLC can be used to separate and to purify proteinspeptides based. Consequently it is often of great interest to ascertain whether two purified proteins interact and how this interac- tion affects their properties. GluX from the rest of the other three other proteins in solution.
I Mixtures of proteins can also be separated by electrophoresis. Other uses such as in foods and pharmaceuticals a high level of purity is required. Separation based on electrophoresis is not acceptable because your power supply is broken.
Electrophoresis is a laboratory technique used to separate DNA RNA or protein molecules based on their size and electrical charge. The proteins used in this technique are separated based on their size and charge. Electrophoresis is used to separate complex mixtures of proteins eg from cells subcellular fractions column fractions or immunoprecipitates to investigate subunit compositions and to verify homogeneity of protein samples.
Given protein can be separated using this method. In polyacrylamide gel electrophoresis proteins migrate in response to an. Samples are loaded into the wells of a gel matrix that can separate molecules by size and an electrical field is applied across the gel.
2D Gel Electrophoresis Two-dimensional gel electrophoresis 2D electrophoresis is a form of gel electrophoresis commonly used to analyze proteins in which mixtures of proteins are separated by two properties in two dimensions on. Because nucleic acids negatively charged ions at neutral or basic pH in aqueous environment this technique is often used to separate DNA or RNA molecules. Both proteins and nucleic acids may be separated by electrophoresis which is a simple rapid and sensitive analytical tool.
So first through anionic detergent SDS you will make the net protein charge as negative then run the electrophoresis and the separation will occurs properly based on. This method is based on the attraction between oppositely charged ions so a cationic stationary phase is used to separate anions in cation-exchange chromatography and an anionic stationary phase is used to separate cations in anion exchange chromatography. How is it possible to use electrophoresis a separation technique depending on molecular charge to separate proteins based on molecular weight.
Usually the proteins are first treated with heat and a chemical called SDS in order to unravel the protein. Method used to separate charged macromolecules of different sizes and to estimate their length. For some applications a crude extract is sufficient.
Proteins are made up of amino acid letters some of which are sometimes charged depending on pH. Use the isoelectric point pI to guide you and your protein of interest on your Ion EXchange chromatography IEX journey. This is the technology behind capillary isoelectric focusing cIEF instrumentation.
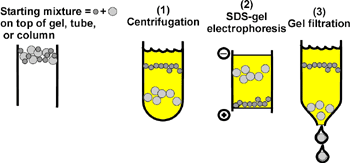
Proteins are heated before being placed in the electrophoresis gel. Two chromatographic methods are frequently used for protein or more often peptide separation. Gel Permeation Chromatography 1-4 Gel permeation chromatography separates protein mole-.
The proteins used in this technique are separated based on their size and charge. A technique that can be used to separate proteins based primarily on the presence of non-polar residues on their surface is called. Chromatography can be used to separate protein in solution or denaturing conditions by using porous gels.
0 Response to "Which Technique Would You Use to Separate Proteins by Charge"
Post a Comment